Central to the design of an ECP are the fundamentals of Electrochemistry as related to producing a given weight of Chlorine. To produce a given weight of Chlorine it is necessary to pass a quantity of DC electricity through a suitable electrolyte and the necessity is to calculate the power requirements and Electrolyser configuration to achieve that requirement.
The calculation to determine the current required to pass through a single electrolytic cell when a potential difference (Voltage) is applied to the Anode and Cathode of that cell is as follows
From Faradays laws of Electrolysis the Amperage required for the generation of a weight of Chlorine from a Sodium Chloride / Water solution would follow.
m = AQ
zF
Where
m = mass generated g
A = relative atomic mass of Chlorine z = Charge number Cl– =1 F = the Faraday Constant = 96,500 C
Q = quantity of electricity C
(C= 1 Coulomb = 1A/second)
Transposing the formula
Q = mzf
A
The data applicable to the process is shown in the following table
| Electrochemical Equivalent | Atomic Mass | Charge Number | |||
| Chlorine | Cl | 1.3228 | 35.4527 | 1 | – |
| Hydrogen | H | 0.37605 | 1.00794 | 1 | + |
Therefore applying the calculation to determine the theoretical amperage requirement at 100% efficiency for 1 g of Chlorine
Q = m z f
A
Q = 1 x 1 x 96500
35.4527
Q = 2721 C
Therefore
To produce 1g of Chlorine by electrolysis requires
2721 C(amp seconds)
3600
0.7558 A/h
Similarly the electrochemical equivalent can be used.
1
0.7558
= 1.3228
The Amp hours that have been calculated above are for a single electrochemical cell to which a potential difference has been applied.
Example 1 illustrates a capacity demand of 24.75kg/h which will be considered as being generated in a stream of 4 electrolysers.
Therefore
24.75 ÷ 4 = 6.19kg/h/Electrolyser
Therefore for this example
Amp/h = 0.7558 A/h x 1000 x 6.19
6.5kg Cl = 4678 Amp/h
This calculated value has to be subject to the Faraday efficiency attributable to the process, values can vary but are typically quoted in the range from 76% to 95% and are related to electrolyte composition and electrode materials. For this example 85% is chosen.
Therefore for 10kg/Cl from a single electrolytic cell
6.19 kg Cl = 4678 Amp/h
0.85
6.19 kg Cl = 5504 Amp/h
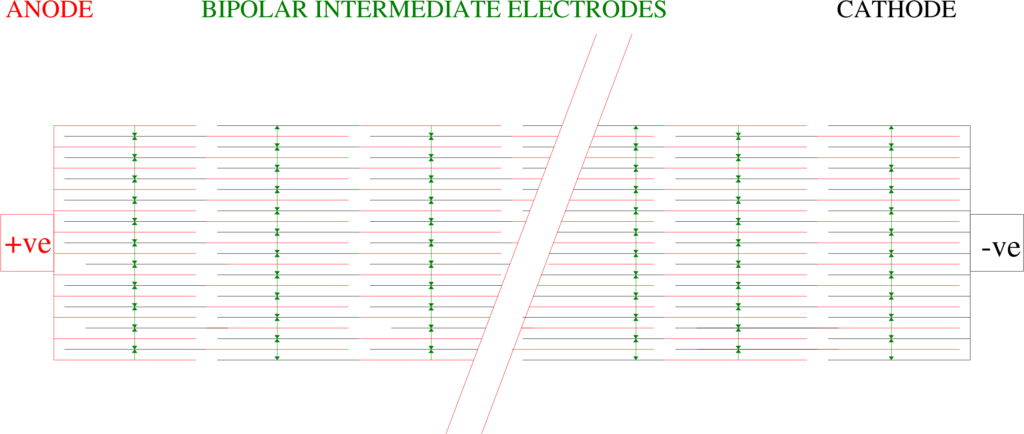
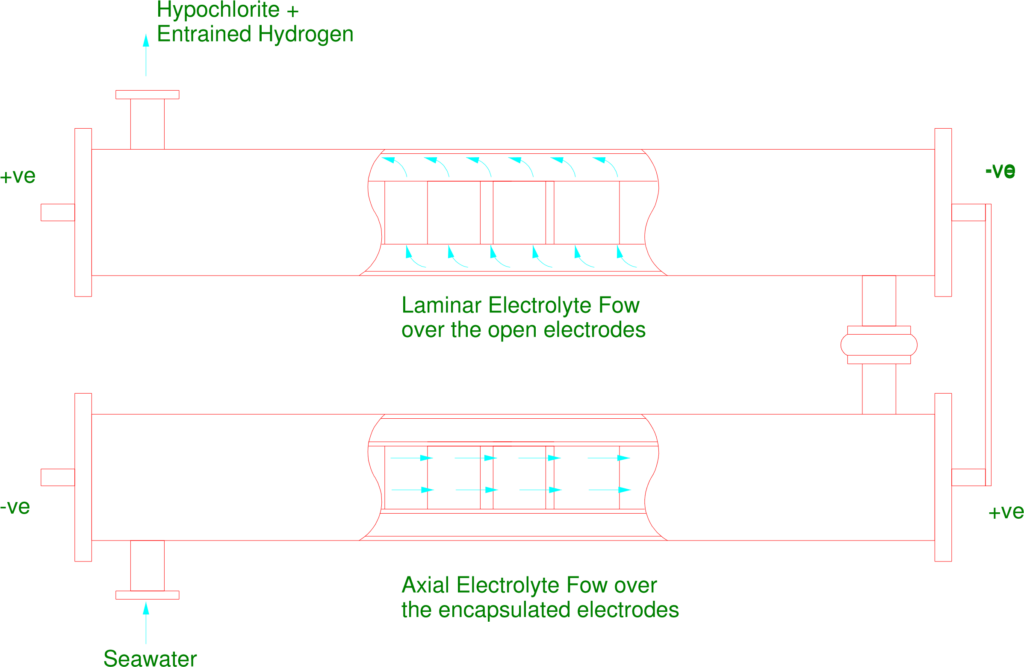
Parallel Plate Electrolysers typically consists of 10 Electrolysis Cells in electrical series, see illustrations below, therefore for 6.19 kg/Cl the amperage to be supplied to the Electrolyser stream would be
5504 ÷ 10 = 550.4 A (DC)
This would be the DC current supplied to the 4 x electrolysers in series to produce the weight determined in Example 1.
NB
A frequently asked question is whether the Sodium Hypochlorite produced by the electrolysis of a brine solution (Seawater) is a Chlorine equivalent. The forgoing calculation demonstrates that it is a direct equivalent.