This course covers explosives atmospheres where the fuel is gas or vapour.
The International Standard for Explosive Atmosphere is IEC 60079.
IEC 60079-20-1 gives data for the properties of gases, some of which will be considered in this unit.
Characteristics of Gases and Vapours #
Properties of a selection of flammable gases to be considered are:
Flammable (explosive) limits, ignition temperature, ignition energy (ignition current), density and flashpoint.
Flammable (Explosive) Limits #
Combustion will only occur if the concentration of the flammable mixture of fuel (gas or vapour) and air is within certain limits. These limits are the ‘lower flammable limit’ (LFL) and the ‘upper flammable limit’ (UFL). Between these limits is the flammable range.
Definition:
L.F.L Concentration of flammable gas, vapour or mist in air, below which an explosive gas
atmosphere will not be formed.
When the percentage of gas by volume is below this limit, the mixture is too weak to
burn, i.e. insufficient fuel and/or too much air.
U.F.L Concentration of flammable gas, vapour or mist in the air, above which an explosive
gas atmosphere will not be formed.
When the percentage of the gas by volume is above this limit, the mixture is too rich
to burn, i.e. insufficient air and/or too much fuel.
An explosive (flammable) atmosphere only exists in the range between these limits (LFL and UFL) and this is called the flammable range.
Different gases or vapour have different flammable limits. The greater the difference between the LFL and the UFL (flammable range), the more dangerous the gas.
Operational safety is generally achieved by operation well below the LFL.
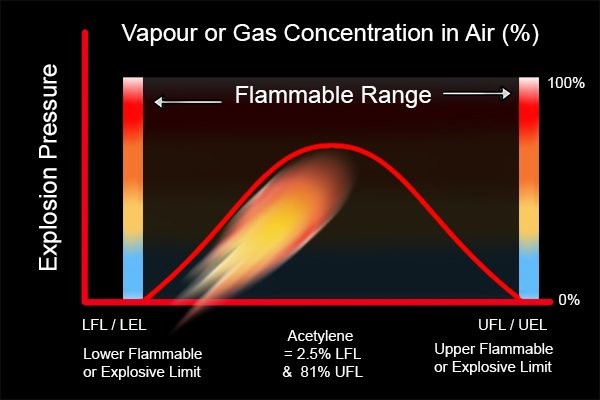
The flammable limit of some materials are given below.
| Material | LEL % by Volume | UEL % by Volume |
| Propane | 1.7 | 10.9 |
| Methane | 4.4 | 17.0 |
| Ethylene | 2.3 | 36.0 |
| Hydrogen | 4.0 | 77.0 |
| Acetylene | 2.3 | 100 |
| Diethyl Ether | 1.7 | 39.2 |
| Kerosene | 0.7 | 5.0 |
| Hydrogen Sulphide | 4.0 | 45.5 |
| Carbon Disulphide | 0.6 | 60.0 |
Ignition Temperature #
The ignition temperature of a material is the minimum temperature, under prescribed
conditions, at which the material can ignite and sustain combustion, when mixed with air at normal pressure, without the ignition being initiated by any spark or flame.
This is also referred to in the standard as the “auto-Ignition temperature”
Ignition temperature is ‘the minimum temperature at which a flammable material will spontaneously ignite’. There is sufficient thermal energy to cause ignition.
Electrical equipment must be selected to ensure that the surface temperature produced by the equipment (indicated by the equipment ‘T’ class) will not exceed the ignition temperature of the flammable atmosphere which may be present around the equipment.
Ignition Energy #
Ignition energy is the spark energy (in joules) which ignites the most easily ignited mixture of the test gas with air at atmospheric pressure.
All flammable (including dusts) have minimum ignition energy (MIE) input required to initiate combustion. The MIE depends on the specific chemical or mixture, the concentration, pressure, and temperature. The MIE’s of a few typical gases are:
| Gas | Ignition energy (µJ) |
| Acetylene | 17 |
| Hydrogen | 17 |
| Ethylene | 70 |
| Propane | 260 |
| Methanol | 290 |
For most materials, the lowest ignition energy value occurs at a concentration about midway between those for the LEL and UEL
Ignition energy is related to the minimum igniting current (MIC), which is an important value in intrinsically safe (I.S) equipment design, and is given in the gas data tables.




